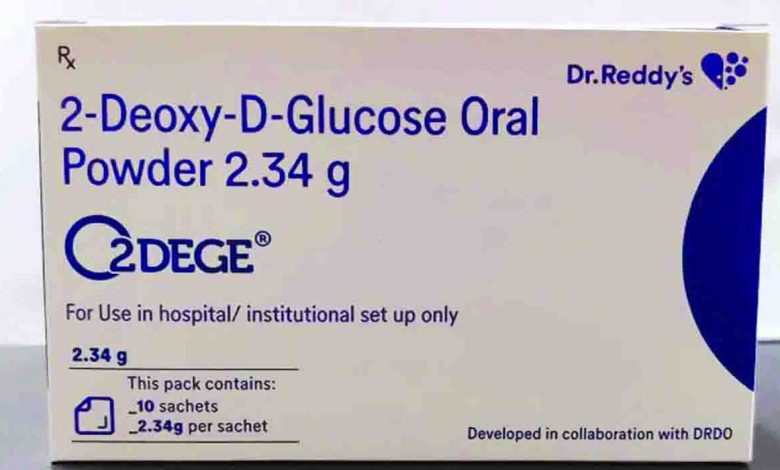
The story behind 2-DG development
Tuesday, 18 May 2021 | PNS | New Delhi
DRDO scientists began working on it in April last year
The DRDO got into the act to develop 2-DG drug way back in April last year when the pandemic hit India. The oral drug is result of more than a year of trial and testing.
Project Director Dr Sudhir Chandana told mediapersons during laboratory tests that his scientists found that the drug was effective in blocking spread of Covid-19 inside the body cells.
“Spurred by these critical findings, we asked the Drugs Controller General of India (DCGI) for permission to conduct clinical trials,” Chandana said.
The DRDO got the go-ahead for the trials in May. The phase two trials were concluded in October last year and final trials in November.
Dr Anand Narayan Bhatt, another scientist associated with this path-breaking project said the rigorous trials took nearly a year before the DCGI approved emergency use of the drug last week.
The INMAS-DRDO scientists conducted laboratory experiments with the help of Centre for Cellular and Molecular Biology (CCMB), Hyderabad.
Giving details about the trials, Bhatt said a substantial number of patients were part of the phase three trials. The tests showed many patients were able to “come out of oxygen (support)”, he added.
On availability of the drug, officials said the hospitals across the country will start getting it by June. At present, the All India Institute of Medical Sciences (AIIMS) Delhi and Armed Forces Medical Services (AFMS) have access to it.
Veterans and serving personnel undergoing treatment for corona at the military hospital in Delhi and for that matter all over the country will be administered the drug as per the doctor’s advice, officials said.